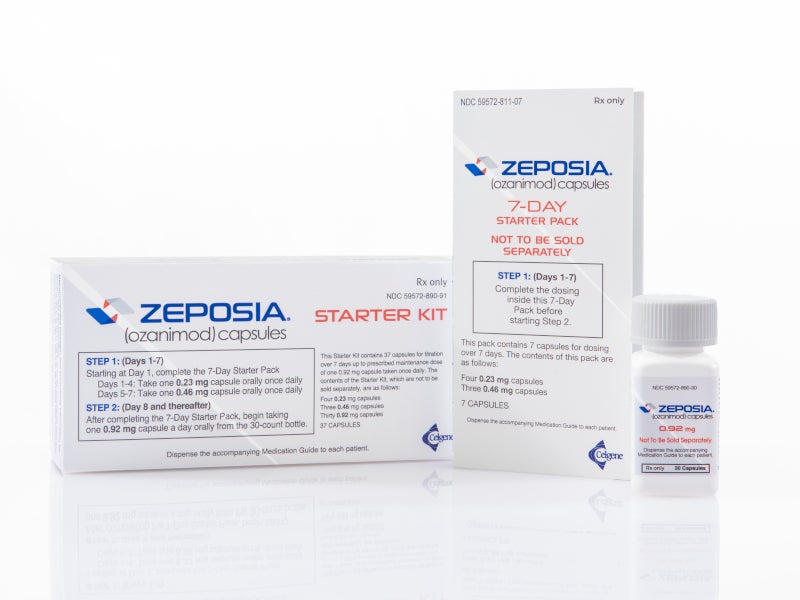
ocrevus start up form
Once youve written a prescription for OCREVUS complete the Start Form or enroll patients online to get them started with OCREVUS. Access PI and Start Resources for HCPs.
Access PI and Start Resources for HCPs.

. Ocrevus may cause unpleasant side effects. Complete entire form and fax to Alongside KESIMPTA at 1-833-318-0680 An incomplete Start Form may delay the start of treatment. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary.
Prescribers first name. When possible you should receive any non-live vaccines at least 2 weeks before you start treatment with OCREVUS. Ad Physicians - View Site to Find Patient Resources For OCREVUS ocrelizumab.
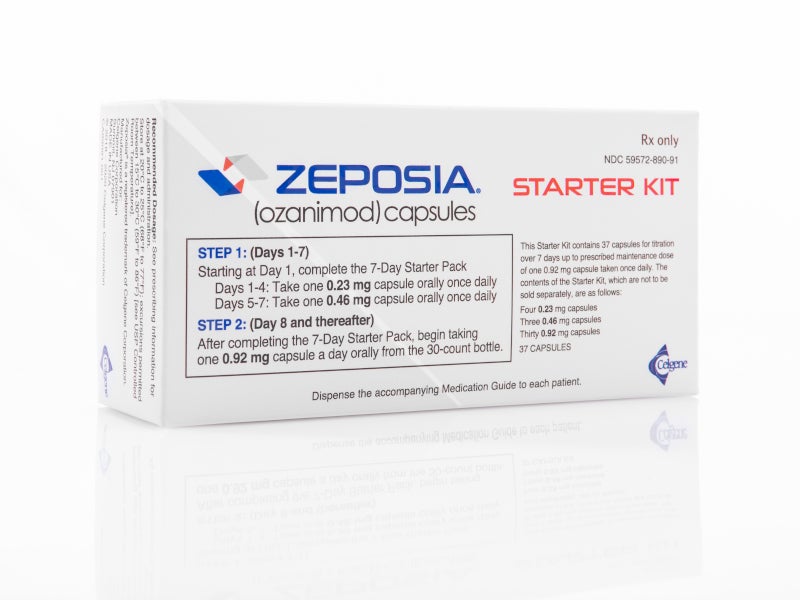
The OCREVUS Start Form is required for enrollment in OCREVUS Access Solutions. RMS and PPMS and their open-label extensions up to approximately 7 years of exposure have shown an association between. If you would like to receive any non-live inactivated.
Ad Physicians - View Site to Find Patient Resources For OCREVUS ocrelizumab. Ad Learn About Genentech Access Solutions Financial Assistance Information For Patients. O Start dose300 mg intravenous infusion followed two weeks later by a second 300 mg intravenous infusion 23 o Subsequent doses.
Prescription Enrollment Form. View full prescribing information and Boxed Warning. 600mg intravenous infusionevery 6 months23.
Ad Learn More About OCREVUS Resources And How Patient Navigators Can Help. Contact Us for Support. Ocrevus ocrelizumab injection is a preservative-free sterile clear or slightly opalescent and colorless to pale brown solution supplied as a carton containing one 300.
Ocrevus may also be used for purposes not listed in this medication guide. Call 1-844-OCREVUS to speak to someone who can answer your questions about OCREVUS and MS and provide. Ad Get patients started with AUBAGIO.
Ocrevus ocrelizumab Fax completed form to 8883021028. Benefits investigation Benefits reverification approximately 6. It must be completed by the provider.
Some side effects may occur during. Ad Learn More About OCREVUS Resources And How Patient Navigators Can Help. OCREVUS is a prescription medicine used to treat.
OCREVUS Start Form Ready to get started. OCREVUS CONNECTS Connecting you with people who can help. The physician portion and submit this completed form.
Ocrevus Order Form Prescriber Signature Date Please Print Name Form 350 NForms300 - PHARMACYF350 - Ocrevus Physician Order Formdocx Orders are initiated unless crossed out. Ad Learn About KESIMPTA. Ad Discover The Safety Efficacy Of TYSABRI.
Contact Us for Support. 2 Patient Authorization and Additional Consents. Ad Learn About KESIMPTA.
The form includes patient insurance and prescription information. Patients first name. View Doctor Resources Downloadable Start Forms.
By completing this form you are requesting services on behalf of your patient which may include. See Website For Safety Boxed Warning PI. Date of birth.
View Doctor Resources Downloadable Start Forms.

Ocrevus Ocrelizumab Ms Infusion Experience

Roche Secures Fda Approval For Ocrevus For 2 Types Of Ms Biopharma Dive Multiple Sclerosis Awareness Roche Fda

Ocrevus Ocrelizumab Ms Infusion Experience

Ocrevus Ocrelizumab Ms Infusion Experience

Design By Pizaz Creative Massage Therapy Business Massage Therapy Massage Marketing
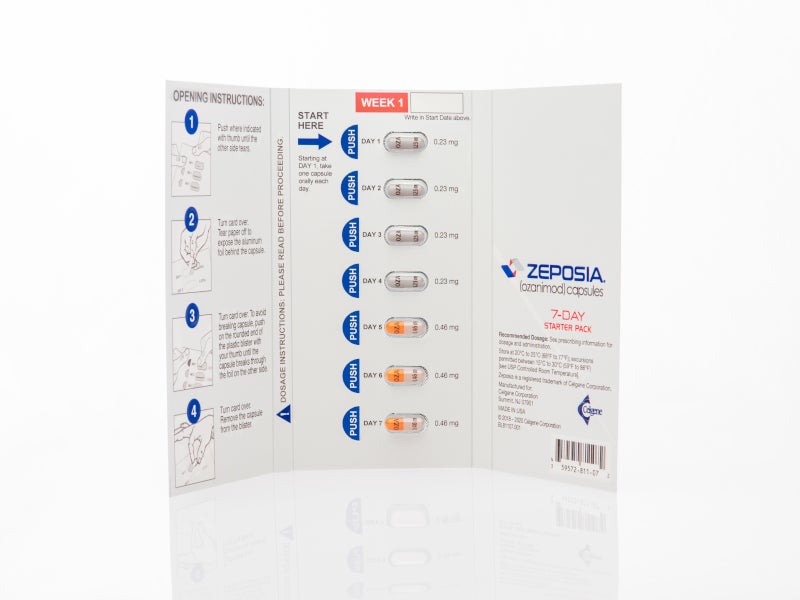
Zeposia Ozanimod For The Treatment Of Multiple Sclerosis

Pin On Multiple Sclerosis To Educate And To Learn

Medical History Form 55 Medical History Health History Form Patient History

Multiple Sclerosis Drug Is First To Dramatically Cut Brain Shrinkage New Scientist
Buy Ocrevus Ocrelizumab Online Price Costs Thesocialmedwork

Primary Progressive Ms Ppms National Multiple Sclerosis Society

Effect Of Tiger Milk Mushroom Lignosus Rhinocerus Supplementation On Respiratory Health Immunity An Respiratory Health Respiratory System Scientific Reports

Multiple Sclerosis Ms Types Symptoms And Causes